Looking at the events of 9/11 requires discipline, keen eye and skepticism about what we are told by the government and relayed through the mainstream media. Many are aware of the obvious contradictions and implausibilities of the official explanations about what happened on 9/11. In this 9/11 Truth cauldron – where official explanations are reviewed and challenged and frequently rejected at face value – the temptation is to gravitate toward another apparent "gotcha" exposing official complicity with 9/11. These weakly supported memes circulate without significant scrutiny -- after all, in a world in which "up-is-down," "war-is-peace" and fake-news is real-news," it is easy to latch onto some simple explanations. One of the simple explanations is that the World Trade Center skyscrapers were destroyed by some type of nuclear device(s) with the key evidence for a nuclear event supported by the presence of certain elements – at supposedly "high" concentrations – in some of the samples.
For example, if a typical person were told that uranium was found at the World Trade Center in concentrations of 3.29 parts per million (ppm), would they know that the concentration of uranium across the United States averages 2.33 parts per million (ppm) and that the concentrations range is 0.24 to 11.0 ppm. It is highly unlikely that typical people would have any frame of reference to evaluate this claim. This article will review the concentrations of 10 elements found at the World Trade Center after 9/11 and provide some perspective on those concentrations by comparing them to the range found in samples across the United States.
Ominous Claims
In the aftermath of the destruction of the World Trade Center on 9/11, the USGS took samples of the dust and analyzed them for the presence of various elements. The results included elements that some people consider exotic, and the presence of those elements was viewed by them as ominous. Furthermore, they claim the presence of elements in the dust, such as uranium and thorium among other elements, “shouldn’t be there.” They conclude that the presence of these elements substantiates a nuclear demolition hypothesis. The following ominous statements have been circulated:
- Barium and Strontium: Neither of these elements should ever appear in building debris in these quantities. The levels never fall below 400ppm for Barium and they never drop below 700ppm for Strontium and reach over 3000ppm for both in the dust sample taken at Broadway and John Streets.[Basis is incorrect]
- Thorium and Uranium: These elements only exist in radioactive form. Thorium is a radioactive element formed from Uranium by decay. It’s very rare and should not be present in building rubble, ever. So once again we have verifiable evidence that a nuclear fission event has taken place.[Wrong]
- Lithium: With the presence of lithium we have compelling evidence that this fission pathway of Uranium to Thorium and Helium, with subsequent decay of the Helium into Lithium has taken place.[Unsubstantiated]
- Lanthanum: Lanthanum is the next element in the disintegration pathway of the element Barium.[Out of Context]
- Yttrium: The next decay element after Strontium, which further confirms the presence of Barium.[Out of Context]
- Chromium: The presence of Chromium is one more “tell tale” signature of a nuclear detonation.[Wrong]
- Tritium: A very rare element and should not be found at concentrations 55 times normal the basement of WTC-6 no less than 11 days after 9/11, which is another “tell tale” sign of nukes.[Out of Context]
While these assertions may sound alarming, they are either incorrect or out of context. A review of the data will show that the element concentrations at the WTC after 9/11 are within the range of expected concentrations based on national and eastern United States surveys of element concentrations. The data shows some exceedances for some elements. Largely, these which are explainable; such as Strontium – which is used in fluorescent lights – and zinc – that was used on tons of galvanized steel that were at the center of the demolition forces. The ratios and concentrations of most of these elements at the WTC can be expected to be found anywhere in North America. Furthermore, the reason for taking multiple samples is to look at trends knowing that individual samples can be significantly affected by small local events – such as the cathode ray tube of a computer monitor crashing near the site where a sample would be collected.
Overview
We will examine the element concentrations at the WTC and show that they are within the range of expected concentrations based on national and eastern United States surveys of elements done by the United States Geological Survey (USGS). There are some elements with concentrations that are higher than “normal” – and they can be explained by the materials within an urban environment and common items that were present in the Twin Towers and other buildings.
USGS Samples From WTC
 Figure 1. Map of lower Manhattan showing (as stacked bar charts) variations in concentration (in percent) of major elemental components of WTC dust and girder coating samples.
Figure 1. Map of lower Manhattan showing (as stacked bar charts) variations in concentration (in percent) of major elemental components of WTC dust and girder coating samples.Immediately after 9/11, the USGS collaborated in an interdisciplinary environmental characterization of the World Trade Center (WTC) area and produced a report, Environmental Studies of the World Trade Center area after the September 11, 2001 attack. A two-person USGS crew collected grab samples from 35 localities within a 0.5 - 1 km radius circle centered on the World trade Center site on the evenings of September 17 and 18, 2001. These samples of dust from the WTC area and from two steel girder coatings from the WTC debris were analyzed to determine composition by major element. The analytical methods employed determined the total concentration (in weight percent or parts per million) of each element in any given sample. As noted in the report, the samples are likely to contain a mixture of different components, such as particles of gypsum, concrete, steel, etc., that together make up the total concentration of elements. Figure 1 shows the location of the samples that were collected.
Two samples were collected from indoor locations that were presumably not affected by rainfall (there was a rainstorm on September 14). Two samples of material coating a steel beam in the WTC debris were also collected. The dust/debris and beam-insulation samples were analyzed for a variety of mineralogical and chemical parameters using techniques such as Reflectance Spectroscopy (RS), Scanning Electron Microscopy (SEM), X-Ray Diffraction (XRD), chemical analysis, and chemical leach test techniques in U.S. Geological Survey laboratories in Denver, Colorado.
The USGS published a table, USGS Chemistry Table, that summarizes data for major elements and all trace elements analyzed in the WTC dust and beam coating samples. Some elements (such as mercury and tin) were not analyzed in these samples. Major elements are listed in percent concentration and trace elements are listed in parts per million concentration. One percent equals 10,000 parts per million.
Figure 2 shows the range of concentrations of the various elements that were found in lower Manhattan. The concentration ranges (colored boxes) and means (horizontal white bars) for major and trace elements in samples of WTC dusts and girder coatings are shown.The blue bar shows the range of the concentrations and the yellow is the mean of the distribution. Ten of the elements are highlighted here. The red line in the graph is the mean of the distribution for the Eastern United states. However, no indication is shown in this slide about the expected range of elements that would be expected. Without information about the variability in the expected samples, there is no way to actually say that the WTC amounts are unexpected.
 Figure 2. Plot showing the concentration ranges (colored boxes) and means (horizontal white bars) for major and trace elements in samples of WTC dusts and girder coatings.
Figure 2. Plot showing the concentration ranges (colored boxes) and means (horizontal white bars) for major and trace elements in samples of WTC dusts and girder coatings.Elements vs. Isotopes
The USGS analysis for lower Manhattan was for elements. No attempt was made to identify isotopes of the identified elements. Isotopes are important because the results of nuclear events, alluded to in the "alarming passages," are unstable atoms which will attempt to stabilize themselves by ejecting neutrons and or protons. These stabilizing ejections of matter occur at characteristics rates and result in changes in the amount of unstable elements that are typically measured in half lives. A half life is the length of time for a group of like elements to decay so that half of the remaining elements remain in the unstable state.
Element Concentration Across the United States
Because the data in Figure 2 does not indicate the range of concentrations, there is little information to assess how far outside the expected range (e.g. outside the expected distribution) a certain sample concentration was. To include an estimated range of concentrations, the distribution data in an older USGS study by Shacklette and Boerngen, Element Concentrations in Soils and Other Surficial Materials of the Conterminous United States (1984 U.S. Geological Survey Professional Paper 1270) was used See Figure 3). This Shacklette and Boerngen analysis summarizes samples that were taken from hundreds of locations across the lower 48 States from 1961 to about 1975. The samples were taken at a depth of approximately 20 cm to capture soils that would have not been subject to agricultural plowing. Results were compiled for 45 elements by developing a geometric mean and geometric deviation to accompany a histogram showing the range of concentrations that were observed.
The geometric mean is calculated by taking nth root of the product of n samples. Finally, a map is provided that shows the location were the samples were taken.
Each element is summarized on a separate page which shows the distributions. Figure 4 shows the results for this element, uranium.
 Figure 4. Uranium Abundance in Soils, Shacklette and Boergen – Shacklette and Boerngen present graphs of element distributions
Figure 4. Uranium Abundance in Soils, Shacklette and Boergen – Shacklette and Boerngen present graphs of element distributionsComparison of USGS WTC Sample vs. Conterminous United States
Given statistical limitations, the best way to assess these distributions is graphically and qualitatively. Traditionally, the way to determine whether a specific sample is within a range is to use a "Student T Test" which estimates the probability that a sample came from the established distribution. However, because the Shacklette and Boerngen data uses geometric mean and geometric deviation, there is no way to develop a similar statistic such as “with an 85 percent confidence we can say that the WTC samples came from national distribution for those respective elements.” Such a statement can be developed with statistics for normal distributions – but not geometric distributions.
On the following figures, the (black and white) image on the right is from Shacklette and Boerngen and the (blue) image on the left is the distribution of the WTC samples for a specific element. The red square on the left graph shows the column where the USGS mean would fall (subject to the resolution of the Excel graph's column width). The green triangle shows the column where the WTC mean would fall (subject to the resolution of the Excel graph's column width). Comparison of elements suggest that samples from the WTC dust are very similar to those expected across the continental United States.
Elements found in comparable concentrations are: Barium, Chromium, Lanthanum, Lithium, Strontium, Thorium, Uranium, Yttrium, Zinc, Iron.
Barium
Shows the concentration of Barium found in dust samples collected from various location in the World Trade Center complex and compares those concentration to what is found in soil samples from across the United States. Barium is used in paint pigments as an additive to enhance whiteness. Additionally, barium was identified in relatively small quantities in the red/gray chips described in Harrit et al and would be part of thermate described in Why Indeed Did the WTC Buildings Completely Collapse? (note: thermate, as a source of barium, may be an interesting area for future investigation / validation).
 Figure 5. Comparison of Concentrations of Barium in Soil and in the WTC Dust– Shacklette and Boerngen
Figure 5. Comparison of Concentrations of Barium in Soil and in the WTC Dust– Shacklette and BoerngenChromium
Shows the concentration of Chromium found in dust samples collected from various location in the World Trade Center complex and compares those concentration to what is found in soil samples from from across the United States.
 Figure 6. Comparison of Concentrations of Chromium in Soil and in the WTC Dust– Shacklette and Boerngen
Figure 6. Comparison of Concentrations of Chromium in Soil and in the WTC Dust– Shacklette and Boerngen
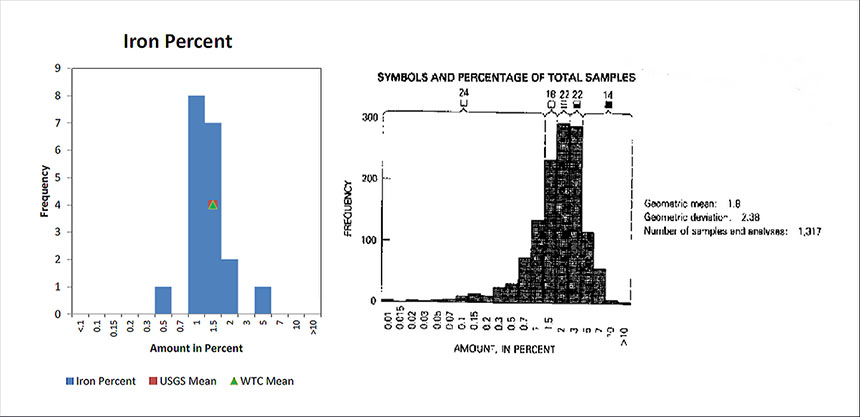
Iron
Shows the concentration of Iron found in dust samples collected from various location in the World Trade Center complex and compares those concentration to what is found in soil samples from across the United States.
Iron concentrations are normal and show that concentrations at the WTC tend to be on the lower side. Some people suggested WTC steel was “dustified” during the demolition on 9/11. If this were the case, the concentration of the iron from the "dustified" steel beams, girders and columns would have been dispersed and included in the WTC samples taken by the USGS. From these results, that are in line with expectations, there is no evidence for “dustification.” Additionally, there are no samples of steel that were observed to have partially dis-appeared. Most of the deformed steel is due to bending and twisting. Some steel was observed to have melted / eroded areas that were observed at small localized points. “Dustitifed” steel would leave large concentrations of iron in the USGS samples – which were not observed.
Note: Higher concentrations of iron were reported in an RJ Lee analysis of dust sample obtained from a gash in the side of the Deutsche Bank Building at 130 Liberty Street as described in Extremely high temperatures during the World Trade Center destruction.
Moreover, the RJ Lee report provides provocative data regarding the abundance of observed iron-rich spheres. A WTC dust sample acquired at 130 Liberty Street shows a “mean of composition” of “Fe spheres” of 5.87% which is very high compared with “Fe spheres” found in ordinary building dust of only 0.04% [1]. As the report notes, the WTC dust has unusual identifying characteristics – in particular, the WTC dust in this sample has nearly 150 times (5.87/0.04) the amount of iron-rich spheres as ordinary dust (where Fe spheres can arise from micrometeorites, for example).
Lanthanum
Shows the concentration of Lanthanum found in dust samples collected from various location in the World Trade Center complex and compares those concentration to what is found in soil samples from across the United States.
 Figure 8. Comparison of Concentrations of Lanthanum in Soil and in the WTC Dust– Shacklette and Boerngen
Figure 8. Comparison of Concentrations of Lanthanum in Soil and in the WTC Dust– Shacklette and Boerngen
Lithium
Shows the concentration of Lithium found in dust samples collected from various location in the World Trade Center complex and compares those concentration to what is found in soil samples from across the United States.
 Figure 9. Comparison of Concentrations of Lithium in Soil and in the WTC Dust– Shacklette and Boerngen
Figure 9. Comparison of Concentrations of Lithium in Soil and in the WTC Dust– Shacklette and BoerngenStrontium
Shows the concentration of strontium found in dust samples collected from various location in the World Trade Center complex and compares those concentration to what is found in soil samples from across the United States. Sources of strontium to account for these elevated results in the 9/11 post-demolition urban environment can be attributed to its presence in florescent lamps and glass. There were a significant number of fluorescent lights and panes of glass at the WTC. The USGS did optical spectrographic analysis to determine the concentration of strontium, however, they provided no information on isotopes. The isotope byproduct of nuclear fission is strontium-90 and the half life of strontium-90 is about 28 years when it trans-mutates into Yttrium.
 Figure 10. Comparison of Concentrations of Strontium in Soil and in the WTC Dust– Shacklette and Boerngen
Figure 10. Comparison of Concentrations of Strontium in Soil and in the WTC Dust– Shacklette and BoerngenThorium
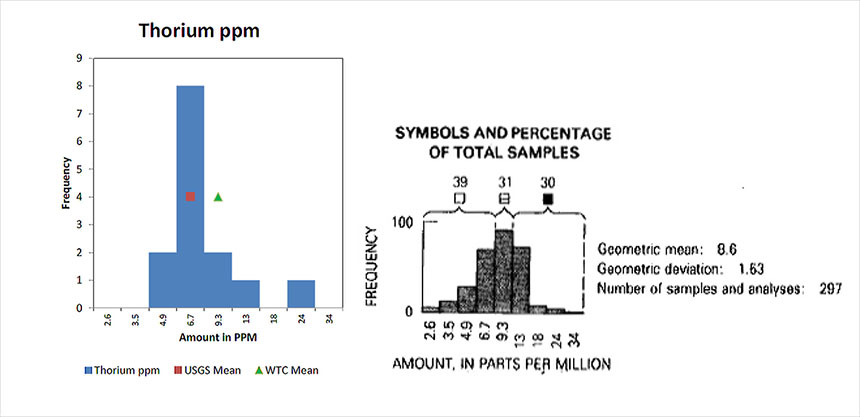
Shows the concentration of Thorium found in dust samples collected from various location in the World Trade Center complex and compares those concentration to what is found in soil samples samples from across the United States.
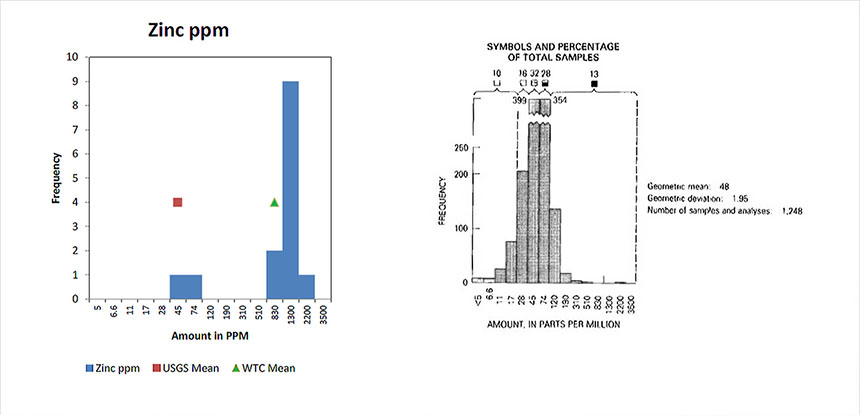
Uranium
Shows the concentration of Uranium found in dust samples collected from various location in the World Trade Center complex and compares those concentration to what is found in soil samples from across the United States.
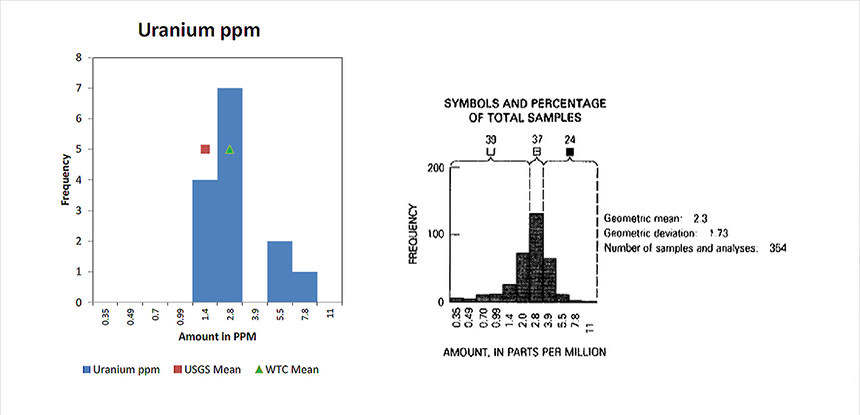 Figure 12. Comparison of Concentrations of Uranium in Soil and in WTC Dust – Shacklette and Boerngen
Figure 12. Comparison of Concentrations of Uranium in Soil and in WTC Dust – Shacklette and Boerngen
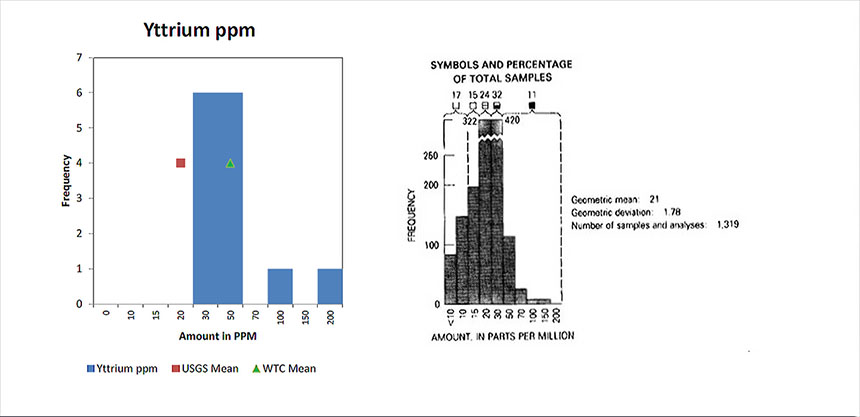
Yttrium
Shows the concentration of Yttrium found in dust samples collected from various location in the World Trade Center complex and compares those concentration to what is found in soil samples from across the United States.
Sources of yttrium that could have created elevated levels in the analysis of the WTC samples come from the use of yttrium in making phosphors, such as the red phosphors used in computer (CRT) monitors and in LEDs. Yttrium is also used in the production of electrodes, electrolytes, electronic filters, lasers various medical applications, etc.
Zinc
Shows the concentration of Zinc found in dust samples collected from various location in the World Trade Center complex and compares those concentration to what is found in soil samples from across the United States.
Zinc, the element used to make galvanized steel is clearly higher than in the national samples. Zinc is as used on tons of galvanized steel that were at the center of the demolition forces.
Cesium
The USGS samples from the WTC site included cesium. The WTC samples contained significantly lower concentration of cesium compared to Eastern U.S. soil samples as shown in Figure 15. Cesium concentrations not documented in the Shacklette and Boerngen paper.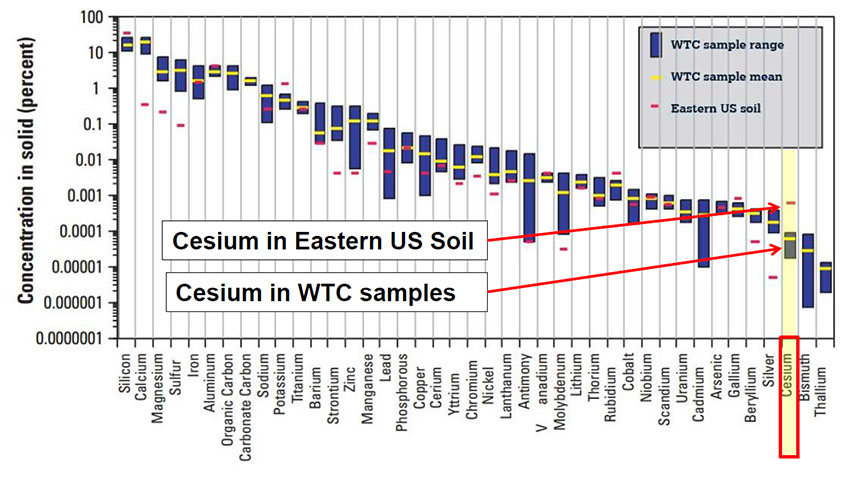 Figure 15. Comparison of Concentrations of Cesium in Eastern US Soil and WTC Dust
Figure 15. Comparison of Concentrations of Cesium in Eastern US Soil and WTC Dust
Tritium
One of the the elements that was studied was the isotope of hydrogen, tritium. The study was performed by Lawrence Berkeley National Laboratory and documented in their report, Elevated tritium levels at the World Trade Center (LBNL et al, 5/14/2002) for the purposes of investigating the release of tritium from manufactured devices. Tritium is manufactured for various purposes such as watch dials, exit signs, night-vision scopes. There were reported to be dozens of night vision scopes in WTC complex with most of these in the US Customs Building, WTC 6.
In the basement of WTC 6 one sample was found to contain 3,530 pC per liter. Excluding WTC 6 basement and sewer samples, the highest value was 210 pC per liter from the surrounding area around Brooklyn, Queens and Manhattan. A violent dispersion would have contaminated large areas with high levels of tritium – not just left the tritium in the WTC 6 basement. A few number of geographically co-located samples are indicative of a local source of contamination. Furthermore, it is known that even deep underground nuclear tests will create very high levels of tritium at the surface.
The levels of tritium that the US Nuclear Regulatory Commission (NRC) considers to be problem range up to, and above, 600,000 pC per liter. These are the concentrations that were observed at the Indian Point Nuclear Station 20 mile upstream from the WTC on the Hudson River. The Indian Point Nuclear Plant had tritium events in 2000, 2001 and other years with the highest recorded tritium levels in the test wells measured at 600,000 pC per liter. While these tritium levels at Indian Point are indicative of a problem, they are over two orders of magnitude greater than the highest WTC 6 reading.
Tritium at another nuclear plant, the now-shuttered Vermont Yankee Nuclear Plant tritium levels below 500 pC per liter were considered to be below measurement capability. While operating, Vermont Yankee site had reading of up to 453,000 pC per liter in various reports (ref1, ref2 , ref3)
Nuclear Detonations Would Have Produced a Brilliant Flash
Nuclear detonations produce such an intense brilliant flash that anyone in lower Manhattan looking directly at the detonating nuclear bomb, even one as small as a, M388, 10 to 20 ton equivalent of TNT nuclear bomb (M388), smaller than the GBU-43/B MOAB conventional bomb that was dropped in Afghanistan, would have blinded people looking directly at the explosion and would have given all others an experience they would never forget.
Below are a series of images captured when an M388 was detonated in the Nevada desert in 1962. See: Declassified U.S. Nuclear Test Film #32. Notice how in Figure 16(a), presented below, that the flash from the detonation of the M388 atomic projectile is so brilliant that the video cam recording the event momentarily malfunctions, until the flash dims significantly, fractions of a second later as seen in Figure 16(b). It should also be appreciated that the camera is positioned over a mile from the point of detonation. If an M388 were detonated at the World Trade Center Complex, even if the light only escaped out of small openings in the building, it would still have been so brilliant that it would have been the most memorable aspect of the destruction that took place on that day. But not one person said anything about seeing an intensely brilliant flash.



We can see in Figure 16(c) that once the brilliant flash subsides the explosion takes on the characteristic of a conventional explosive. This is likely the reason people jump to the conclusion that a small tactical nuclear detonation resembles what we all saw in the countless videos taken on 9/11, That, however, discounts the fact that a nuclear explosion is accompanied by a brilliant flash.